Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is
Par un écrivain mystérieux
Last updated 07 juin 2024

Molar heat capacity (left scale) and thermal conductivty (right scale)

Consider the following reaction: C (graphite) +O_2( g) →CO_2( g): ΔH=-x_1calC (diamond) +O_2( g

Compare Enthalpy Change of Formation of CO and CO2

5.7: Enthalpy Calculations - Chemistry LibreTexts

PPT - Chapter 14 Heat, Work, Energy, Enthalpy PowerPoint Presentation, free download - ID:4284761
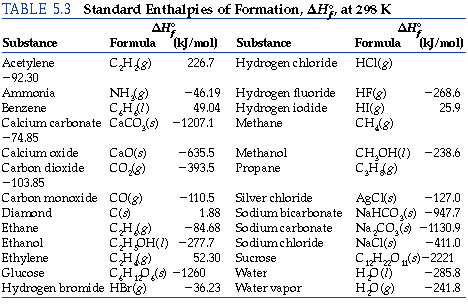
Calculate the enthalpy of reaction for the reaction CH_3COOH + H_2O -> CH_3CH_2OH + O_2?

Effects of CO2 and N2 Dilution on the Combustion Characteristics of H2/CO Mixture in a Turbulent, Partially Premixed Burner
Solved] PLEASE HELP. ORGANIC CHEM 1. 2 3. 4. 5. 6. 7. 8. 9. 10. 11. 12..

Compare Enthalpy Change of Formation of CO and CO2

Full article: CeO2-based oxygen storage capacity materials in environmental and energy catalysis for carbon neutrality: extended application and key catalytic properties
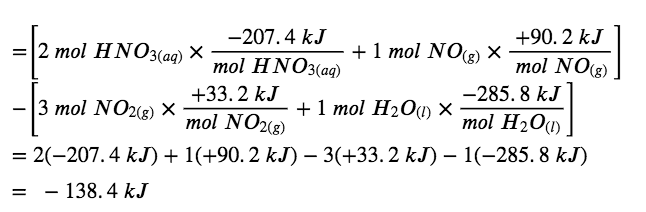
3.6 – Hess' Law – General Chemistry for Gee-Gees
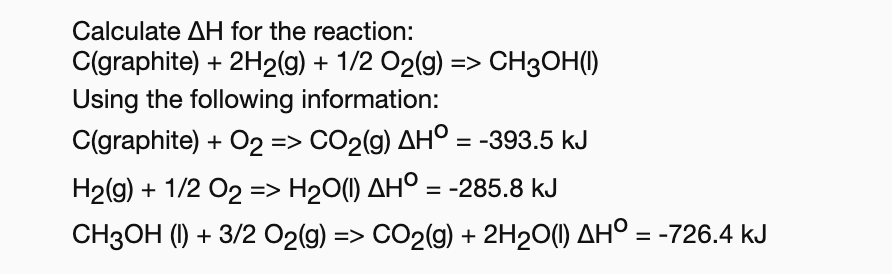
Answered: Calculate AH for the reaction:…

1st PUC Chemistry Question Bank Chapter 6 Thermodynamics - KSEEB Solutions
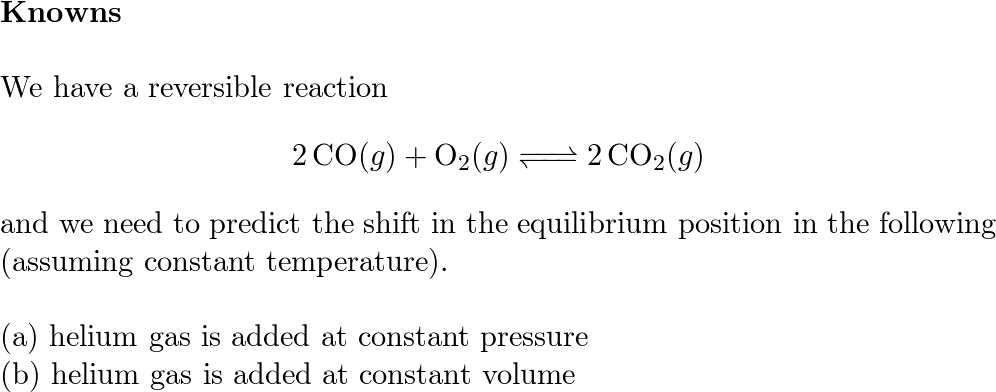
Consider the gas-phase reaction 2 CO (g) + O2 (g) <=> 2 CO2
Recommandé pour vous
 Gants chauffants G-Heat: pour ne plus avoir froid! - Trail & Running14 Jul 2023
Gants chauffants G-Heat: pour ne plus avoir froid! - Trail & Running14 Jul 2023 Gants chauffants confort + Batterie - G-HEAT - Loisir-Plein-Air14 Jul 2023
Gants chauffants confort + Batterie - G-HEAT - Loisir-Plein-Air14 Jul 2023 Gants de travail chauffants en cuir - G-HEAT14 Jul 2023
Gants de travail chauffants en cuir - G-HEAT14 Jul 2023 G-Heat ®14 Jul 2023
G-Heat ®14 Jul 2023 Batterie G-Heat pour vestes et gilets de travail chauffants14 Jul 2023
Batterie G-Heat pour vestes et gilets de travail chauffants14 Jul 2023 G-Heat : Le gilet rafraîchissant14 Jul 2023
G-Heat : Le gilet rafraîchissant14 Jul 2023- Avis de G Heat Lisez les avis marchands de www.g-heat.com14 Jul 2023
 Olympia Heating and Cooling Services - G & G Heating14 Jul 2023
Olympia Heating and Cooling Services - G & G Heating14 Jul 2023 G-HEAT - Gants Chauffants Confort - Mixtes - Tactiles - Résistants14 Jul 2023
G-HEAT - Gants Chauffants Confort - Mixtes - Tactiles - Résistants14 Jul 2023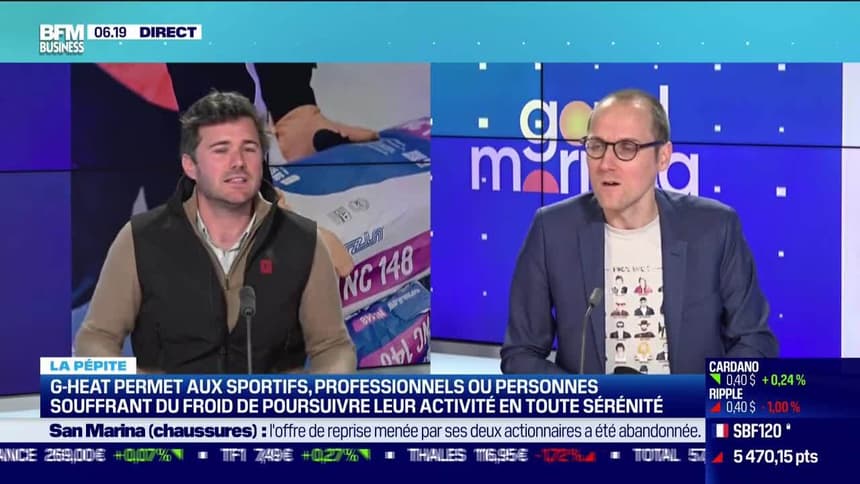 La pépite : G-Heat développe des vêtements technologiques14 Jul 2023
La pépite : G-Heat développe des vêtements technologiques14 Jul 2023
Tu pourrais aussi aimer
 TP-Link Deco BE85 Review: Too Much, Too Soon14 Jul 2023
TP-Link Deco BE85 Review: Too Much, Too Soon14 Jul 2023- VISSEUSE ELECTRIQUE FILAIRE - ATEC - ATEC14 Jul 2023
 BOENMED: Les fabricants de produits médicaux de haute qualité14 Jul 2023
BOENMED: Les fabricants de produits médicaux de haute qualité14 Jul 2023.jpg) EDGAR - Coussin 45x45 cm avec housse de coussin en 85% polyester recyclé - Eco Line collection - Charcoal Gray - anthracite, Coussin14 Jul 2023
EDGAR - Coussin 45x45 cm avec housse de coussin en 85% polyester recyclé - Eco Line collection - Charcoal Gray - anthracite, Coussin14 Jul 2023 Boîte publicitaire carton 100% personnalisable14 Jul 2023
Boîte publicitaire carton 100% personnalisable14 Jul 2023 Panier Effet Rotin Respirant Style Lit pour Chat • Le palais du rotin14 Jul 2023
Panier Effet Rotin Respirant Style Lit pour Chat • Le palais du rotin14 Jul 2023 Bubble Wrap® - Hollinger Metal Edge14 Jul 2023
Bubble Wrap® - Hollinger Metal Edge14 Jul 2023 TP-LINK ROUTEUR WiFi sans fil MU-MIMO AC1200 (ARCHER C64)14 Jul 2023
TP-LINK ROUTEUR WiFi sans fil MU-MIMO AC1200 (ARCHER C64)14 Jul 2023 idées de cadeaux foot14 Jul 2023
idées de cadeaux foot14 Jul 2023 Lot de 4 balles Anti-Stress colorées Fidget Balls14 Jul 2023
Lot de 4 balles Anti-Stress colorées Fidget Balls14 Jul 2023

